As certification and inspection systems grow in complexity, scale, and speed, traditional models of human-led governance are increasingly strained.
Read MoreNews
A QTICS Automotive Zrt. tulajdonosi köre – a QTICS Group, a SAASCO Tanácsadó és Mérnöki Iroda, valamint a QFD Group – sikeresen megvásárolta a csaknem 75 éves szakmai múlttal rendelkező JÁFI-Autókut Kft.-t.
Read MoreThe shareholder group of QTICS Automotive Zrt. – comprising QTICS Group, SAASCO Consulting and Engineering Office, and the QFD Group – has successfully acquired JÁFI-Autókut Kft.,
Read MoreType testing—more commonly called type approval—is the make-or-break moment in a vehicle or component’s life.
Read MoreIf you take a close look at the parts of your car, especially the lamps, windows, mirrors or even the seat belts, you might notice a small circle with the letter E inside, followed by a number.
Read MoreArtificial intelligence is transforming the automobile from a largely mechanical product into a dynamic, software-driven platform.
Read MorePart Two of Our Interview with Zoltán Karászi, CEO of QTICS Group
Read MoreInterview with Zoltán Karászi, CEO of QTICS Group
Read MoreRecently, QTICS Automotive, in partnership with ATIC, successfully facilitated the completion of over 400 kms of real-world driving reliability tests for the BYD T6 model vehicle in Hungary.
Read MoreIn his presentation at the MedTech Minds conference at Pécs, Attila Juhász, consultant at SAASCO Ltd. delved into the global procedures for introducing medical devices to the market. - Part 2
Read MorePéter Mátyus, a seasoned engineer in the automotive industry, has recently embarked on a new chapter at QTICS Automotive Plc.
Read MoreTogether, we aim to enhance conformity assessment and regulatory compliance in the automotive industry, leveraging ATIC’s extensive expertise and global reach...
Read MoreIn his presentation at the MedTech Minds conference at Pécs, Attila Juhász, consultant at SAASCO Ltd. delved into the global procedures for introducing medical devices to the market.
Read MoreWhat challenges does medical AI development face today? Where does the untapped potential lie? In his presentation at this year's MedTech Summit Budapest conference, futurologist Dr. Rab Árpád discussed his experience in medical AI developments.
Read MoreInterjú Prof. dr. Haidegger Tamással az Óbudai Egyetem, Egyetemi Kutató és Innovációs Központ (EKIK) főigazgatójával a 2024-es Lehetőségek és kihívások konferencia kapcsán - 1. rész
Read MoreInterjú Prof. dr. Haidegger Tamással az Óbudai Egyetem, Egyetemi Kutató és Innovációs Központ (EKIK) főigazgatójával a 2024-es Lehetőségek és kihívások konferencia kapcsán - 2. rész
Read MoreA COVID járvány bebizonyította, hogy az egyéni védőeszközök helytelen használata fokozott kockázatot jelent, növeli a keresztfertőzések rizikóját is.
Read MoreInterjú Dr. Palicz Tamással, a a Semmelweis Egyetem Egészségügyi Menedzserképző Központjának stratégiai igazgatóhelyettesével
Read MoreAz orvostechnikai eszközökre vonatkozó szabályokat tartalmazó új rendelet (MDR) egyik kiemelkedő változtatása a korábbi szabályozáshoz képest, hogy szigorúbb követelményeket fogalmaz meg a gyártók számára...
Read MoreHigh-end cars are no longer exclusive for new auto tech. In 2023, affordable models with safety advances emerge. We explore new ideas paving auto's next gen. Watch for these features on your next car.
Read MoreInterview with Zoltán Karászi, Chairman of QTICS Group
Read MoreInterjú Karászi Zoltánnal, a QTICS Group elnökével.
Read MoreMedical breakthroughs this week from targeted gene therapy to nanorobots
Read MoreThe transportation industry is on the cusp of a groundbreaking transformation as the development of robotaxis gains momentum.
Read MoreThe article discusses the European Union's regulations on medical device performance and safety, also mentions the development of the EUDAMED database, aimed at enhancing traceability and transparency in the medical device industry.
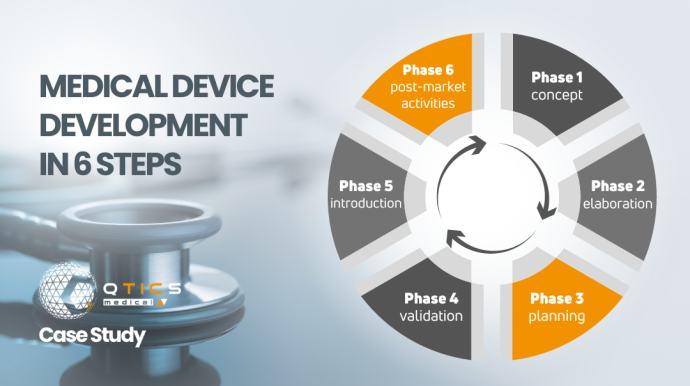
Read MoreOur case study shows the 6 steps manufacturers need to go through to get their medical device on the market.
Read MoreGroundbreaking Medical Innovations This Week: Pioneering Advances in Diagnostics and Gene Therapy
Read MoreAndrás F. Tóth, Leader of QTICS Medical Division shared his experiences on MedTecLIVE, which is one of the most important trade fairs for the German medical technology industry.
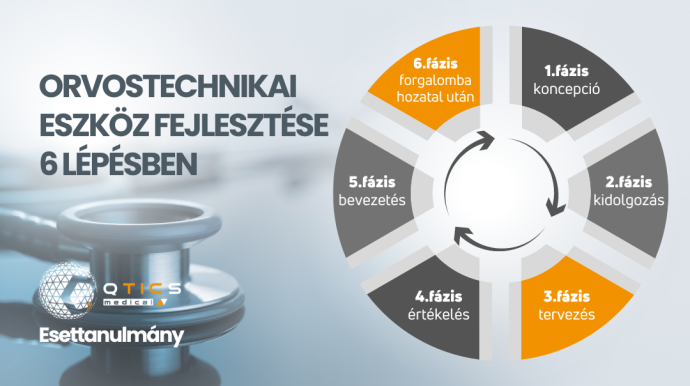
Read MoreEsettanulmányunkból kiderül, hogy melyik az a 6 lépés amin a gyártóknak végig kell menniük, hogy orvostechnikai eszközük forgalomba kerülhessen.
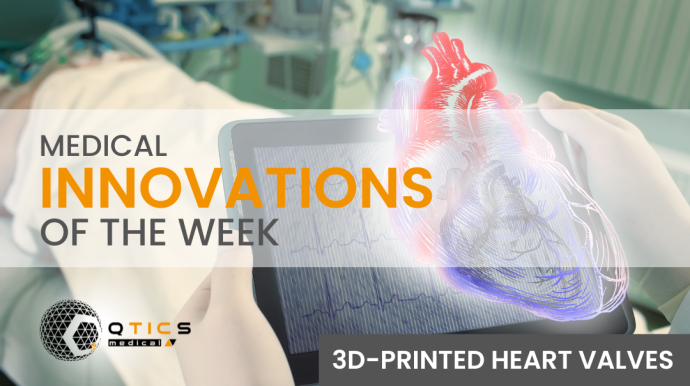
Read MoreA portable brain scanner, antibody treatment for COVID-19, and 3D-printed heart valve are the most important medical innovations of the week.
Read MoreEsettanulmányunk bemutatja, hogy egy MDR bevezetési projektben a fejlesztőmérnököknek milyen tennivalóik vannak a "Design freeze" szakaszban, illetve hogyan lehet gördülékenyen lebonyolítani a megfelelőségértékelési eljárást.
Read MoreEgész napos, interaktív workshopunkon azokról a digitális trendekről és lehetőségekről lesz szó, amelyek segítségével látványosan javítható az egészségügyi szolgáltatások felhasználói élménye és hatékonysága.
Read MoreA hazai egészségügyi piac egyik legjelentősebb vállalatának minőségirányítási vezetője mesélt nekünk az MDR átállás sikeres teljesítéséről és a folyamat során tapasztalt kihívásokról.
Read MoreA sikeres MDR szerinti CE jelölés megszerzéséhez –ugyanúgy, mint egy sikeres filmhez– kell egy jó forgatókönyv.
Read MorePéter Rácz, Sales Leader of QTICS Group shared his experiences on Istanbul's biggest medical device related exhibition, Expomed Eurasia.
Read MoreTravelogue of the Medical Division's week-long visit to Seoul.
Read MoreAz MDR/IVDR átállás során a gyártók számára egyik legnagyobb kihívást a „State of the art” szabványoknak való megfelelés igazolása jelenti, melynek része a jó használhatóságra vonatkozó követelmények teljesítése is. Lássuk, miért olyan fontos ez..
Read MoreThe previously approved regulation -stating that the MDR deadline will be extended, has now entered into force. Take a look at a short summary of the most important points of the regulation.
Read MoreAz orvostechnikai eszközt gyártó cégek tudnak-e elég gyorsan reagálni azokra a kihívásokra, melyeket az új szabályozási környezet és alkalmazott szabványok állítanak eléjük? Esettanulmányunk egy kiváló példa a rugalmas alkalmazkodás fontosságára.
Read MoreA hazai orvostechnikai iparág egyik legjelentősebb szakmai rendezvénye, a Lehetőségek és Kihívások konferencia immár 5. alkalommal került megrendezésre hazánkban.
Read MoreThe Testing, Inspection and Certification (TIC) sector is a rapidly evolving industry that is constantly adapting to changing market needs and regulatory requirements. Here are the newest and most important changes in the TIC sector.
Read MoreKépzésünk célja, hogy az orvostechnikai eszköz gyártóknak átfogó képet adjon arról, hogy milyen lépések szükségesek az egészségbiztosítási (NEAK) finanszírozás rendszerébe történő bekerüléshez.
Read MoreMDR átállás jelenlegi, de még nem kihirdetett végső határideje: 2028.12.31., amely során komoly követelményeknek kell eleget tenniük az orvostechnikai eszköz gyártóknak. Ehhez nyújtunk segítséget!
Read MoreA rendezvény célja, hogy az egészségiparban és orvostechnikában dolgozókat tájékoztassa a nemzetközi innovációkról, szabályozási környezetről, fejlesztési forrásokról, illetve a hatósági eljárások aktualitásairól.
Read MoreISO 13485 is considered the quality bible of the medical device industry all over the world.
Read MoreWe live in a technologically dependent generation in which development is so fast that their impact on our health and safety is almost untraceable
Read MoreA new study unveiled a small, soft, flexible implant that relieves pain on demand and without the use of drugs. The device could provide a much-needed alternative to opioids and other highly addictive medications.
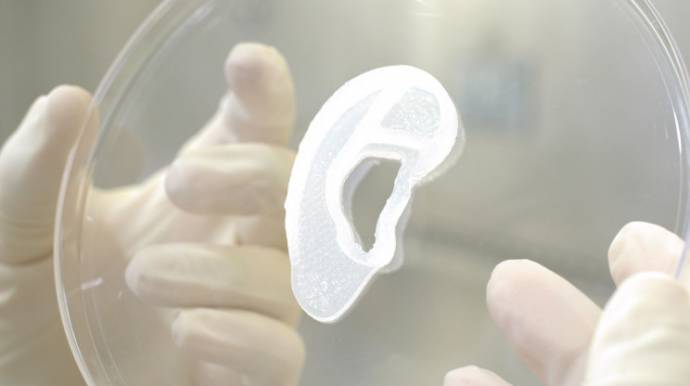
Read MoreA firm has successfully transplanted a printed ear made from a patient’s stem cells with the help of biological 3D printing.
Read MoreLabelling in IEC 60601-1 ed3.1 for medical devices
Read MoreFrom May 27, 2022 00:00 am it is compulsory to apply Regulation (EU) 2017/746 IVD (Regulation on In Vitro Diagnostic Medical Devices).
Read MoreArtificial intelligence enables medical device manufacturers to predict quality problems before they occur. Can AI help you improve your operations?
Read MoreWhat are the main challenges and weaknesses of IoT systems that would require thorough testing?
Read MoreDrones or UAV (Unmanned Aerial Vehicles) are a type of aircraft that flies without a pilot on board
Read MoreMore than 4 years ago, SAASCO Kft. organized the first conference on medical devices technology in Hungary.
Read MoreQTICS Medical Service Group, alone with SAASCO and MediKlaszter, is proud to have reached another milestone with the 4th Opportunities and Challenges Conference
Read MoreA legnagyobb hazai orvostechnikai konferencia. Iránytű az orvostechnikában és az egészségiparban.
Read MoreDigital technologies are gradually transforming the relationship between doctor and patient. In England, general practices are expected to be available to patients online.
Read MoreWhat exactly is digital medicine? This field involves the use of digital tools in the service of human health.
Read More