Why is CE marking important? Why do these two letters have to be placed on a medical device at all? Of course, these questions are mostly asked by "start up" companies. There are basically two concepts to answer this question. CE marking certifies the effectiveness and safety of medical devices! For medical devices, efficacy and safety are particularly important as the stakeholders are diverse, including healthcare workers, healthcare providers, governments, industry, patients and ordinary people.

In the medical devices market, it is essential to ensure that product development meets all regulatory requirements. Understanding and taking into account complex clinical and regulatory requirements early in the product lifecycle can ensure that a company can become competitive by reducing time to market.
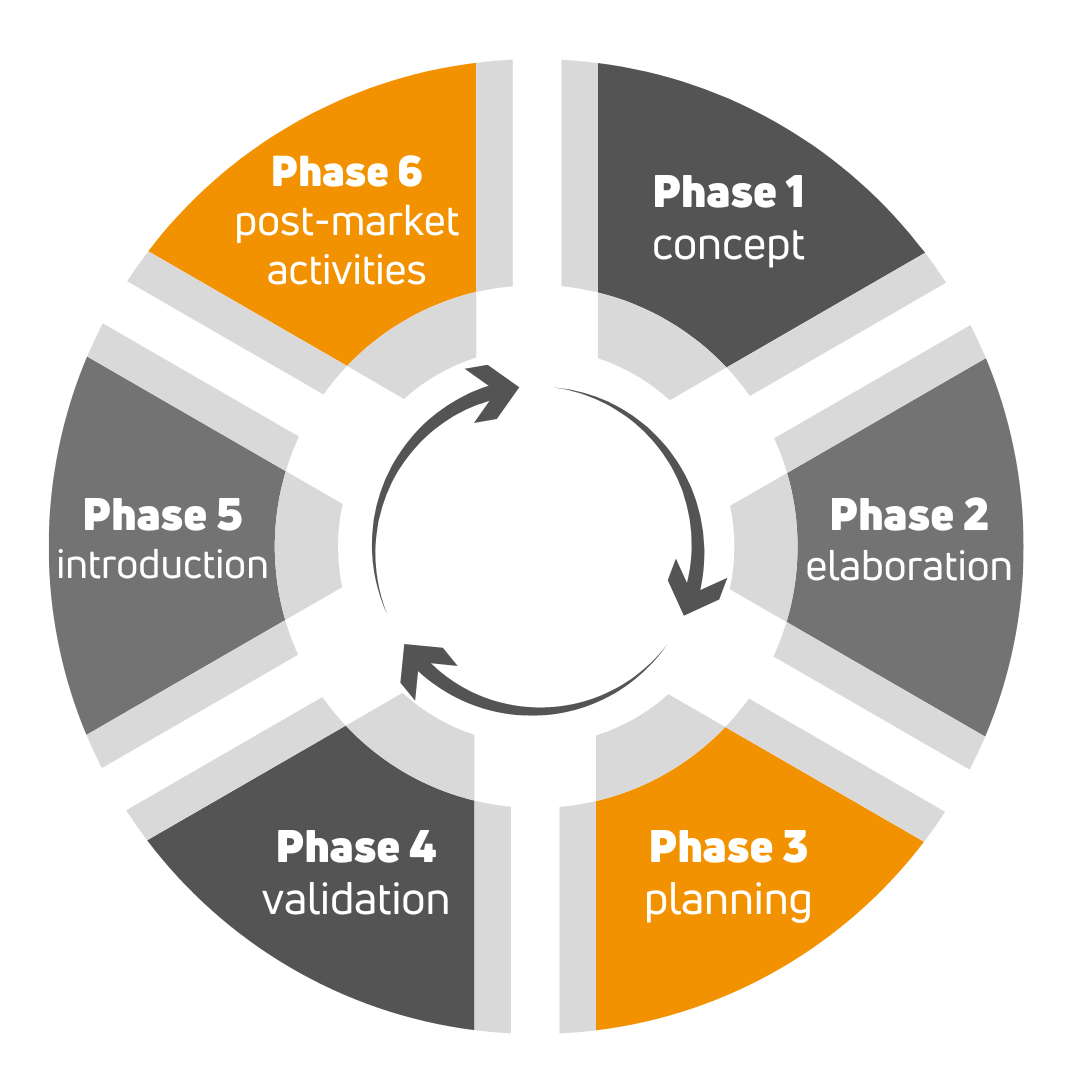
In order to get a CE marked medical device into the store market, there are essentially 6 steps to go through, from idea, through production-ready prototype, to a satisfied and cured patient.
Phase 1 involves identifying the users of the medical device and their needs, defining the intended use of the device. In practice, a preliminary concept must be drawn up by the manufacturer. QTICS Medical will help you to establish the regulatory requirements by providing consultations and training.
In phase 2, the design inputs must be defined based on user needs and technical requirements. It is the manufacturer's responsibility to refine the concept, produce the requirements documents. QTICS Medical's expert team will prepare the Regulatory Strategy for the manufacturer at this stage, including the project plan for the introduction of CE marking under the MDR, but we can also provide preliminary safety laboratory testing.
The Design phase does the lion's share of the work. This is the phase where the safety testing of the medical device has to be carried out, the design and development documents have to be prepared, and the product design and manufacturing processes have to be developed. QTICS Medical can help you to set up a quality management system, prepare the complete MDR/IVDR technical documentation, but our testing laboratories can also perform the necessary pre-clinical safety studies.
In phase 4, the final validation of the medical device and manufacturing processes is carried out, and if necessary, clinical trials are conducted. Our clinical experts will conduct the clinical trial, if required, based on the clinical trial strategy. On the other hand, with the assistance of our consultants, we help finalise the label and instructions for use, support the registration in the Eudamed database.
In the Introduction phase, the conformity assessment procedure and the product launch are carried out. We support the manufacturer during the on-site audit of the notified body.
The last, phase 6, is the post-market surveillance activity. This is where customer complaints and serious adverse events are handled. Besides maintaining the quality management system and technical documentation, QTICS Medical also prepares the necessary PMS and PMCF plans and reports.
To sum up, developing a medical device and obtaining CE marking is not easy, just as it is not easy to climb a mountain peak thousands of meters high. However, if you take an oxygen tank, a map and sherpas to help you, you have a better chance of reaching the top of the mountain. Therefore, we encourage everyone to use the services of QTICS Medical to help you complete this 6-step hike.
